CTMS
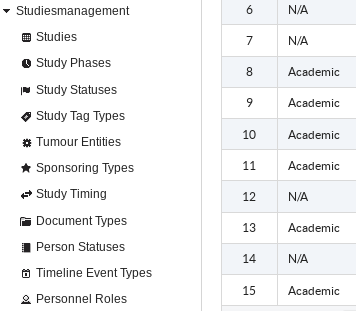
Clinical Trial Management System helps with management of administrative clinical trials data in mono and multi-centre research projects. It allows tracking of studies statuses and deadlines and also documenting the responsibilities of study personnel and organizations roles within trials.
EDC
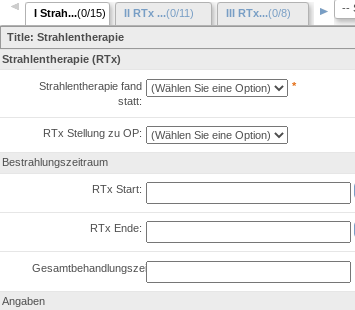
RPB integrates with Electronic Data Capture system which is used to build eCRF forms and conduct study data. RPB provides features to semantically annotate EDC eCRF fields in order to allow the generic access to data captured within the forms.
PACS

Script-able research PACS system is used to store all documented medical imaging and treatment planning data in DICOM format. All data is pseudonymised according to DICOM supplement 142 (Clinical trial de-identification).
LAB
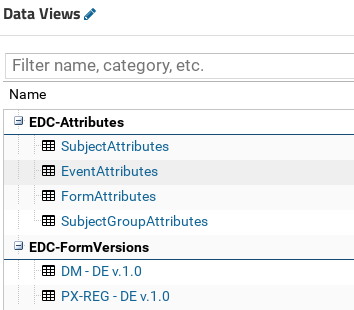
Generic and instrument specific laboratory assay data or experimental data is stored in tabulated form within projects file repositories together with data dictionaries necessary for evaluation. Such organised datasets can be kept within the scope of authoring site or moved to shared location accessible for all sites in multi-centre study.
PID
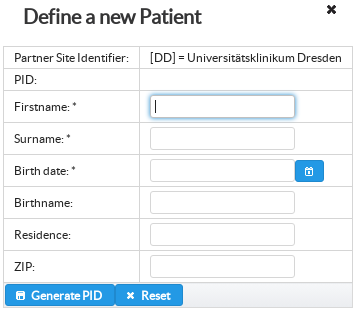
Patient in RPB can be represented with patient unique pseudonyms generated via site specific pseudonymisation service. In order to guarantee unique pseudonyms across multiple partner sites RPB prefixes patient pseudonyms with global RPB partner site identifiers.
DICOM-RT
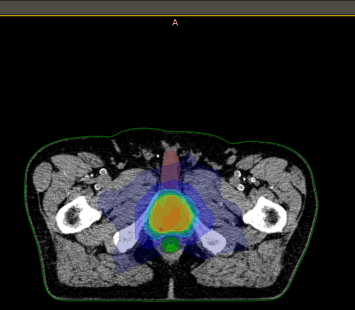
Radiotherapy treatment planning data can be queried and visualised in tree like view showing the planning imaging, delineated RT structure set and corresponding RT plan and RT dose DICOM instances locally presentable via standalone DICOM-RT viewer