Application suite for translational research
The RPB platform is a web-based solution which supports collection and exchange of radiotherapy specific research data in large scale multi-centre clinical and pre-clinical studies. It delivers a study management and electronic data capture system with special extensions dedicated to secure upload of medical imaging and treatment plans in DICOM format.
These tools allow the trial and data management units to handle multi-modal data conduction activities that yield to finalised patient cohort datasets. The domain model of the platform is build around radiotherapy research field and allows linkage of data across the platform integrated subsystems and databases.
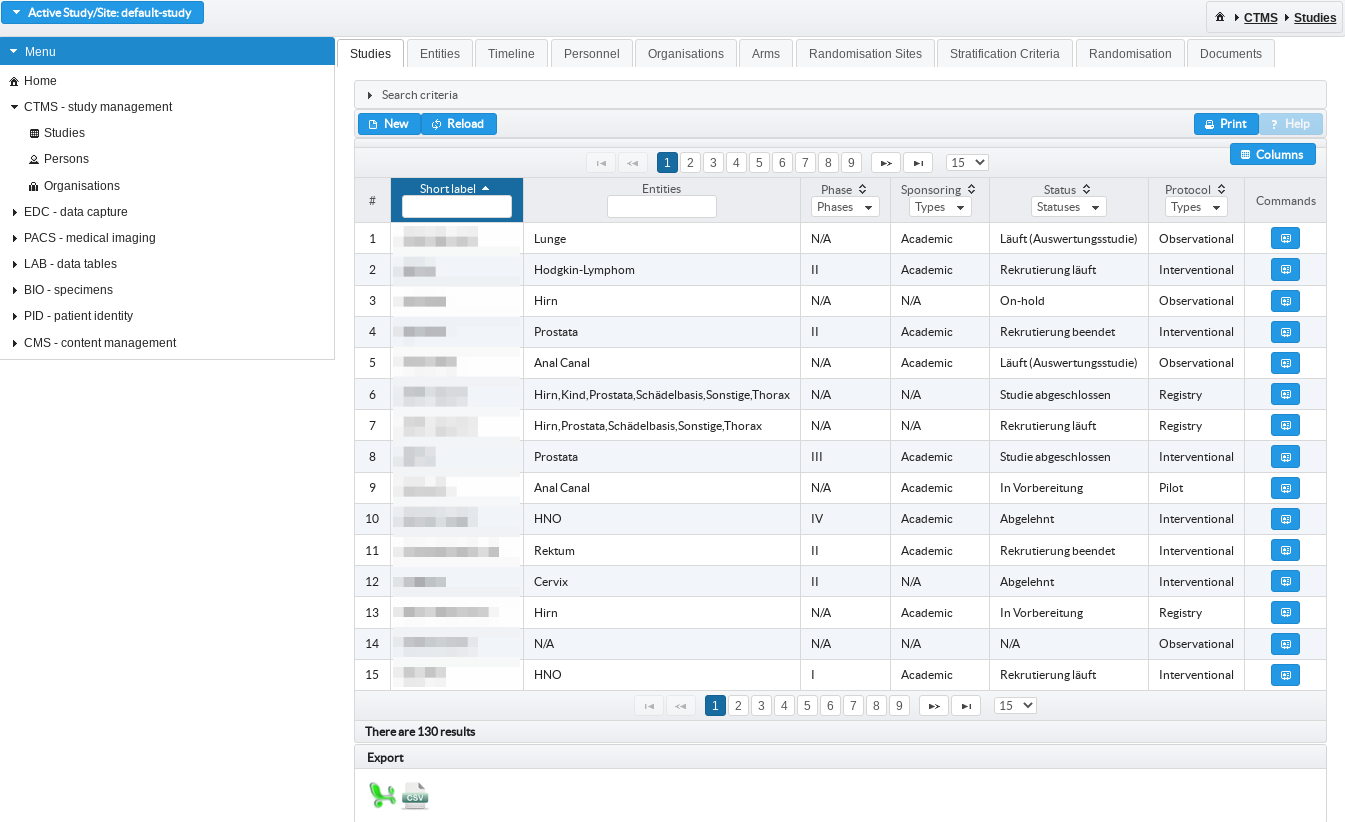
Managing multi-centre clinical trials
Studies with personnel, organisations and project timeline can be created and configured for variety of trial conduction protocol.
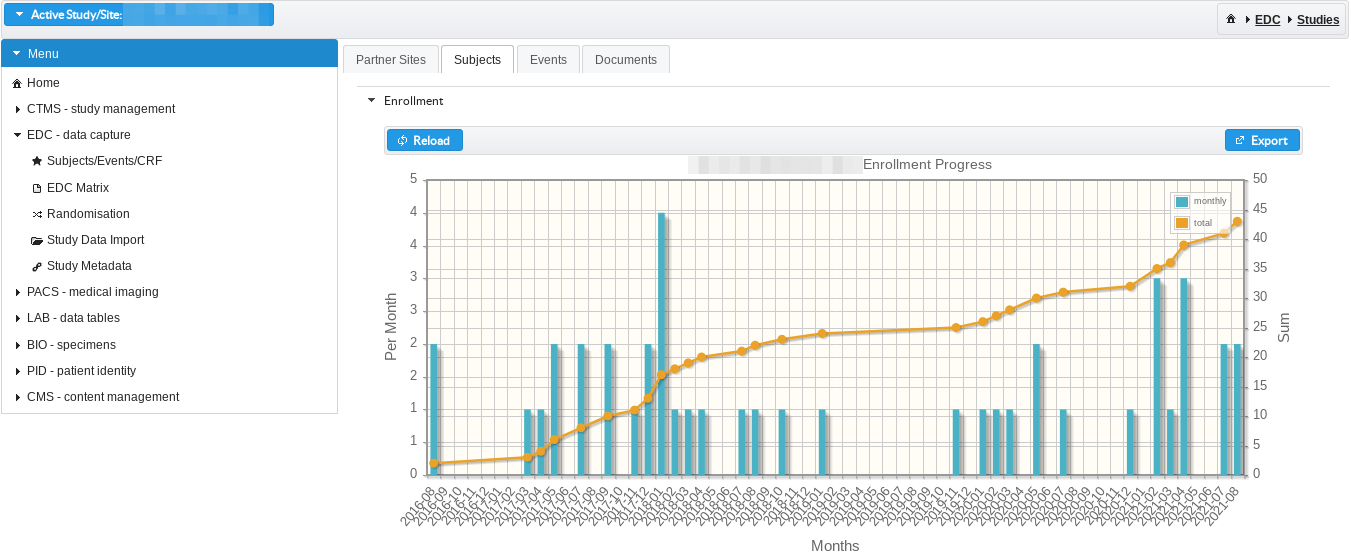
Subjects de-identification and study subjects enrollment
Subjects can be uniquely de-identified prior inclusion into specific study and enrollment performance can be tracked per project and study site.
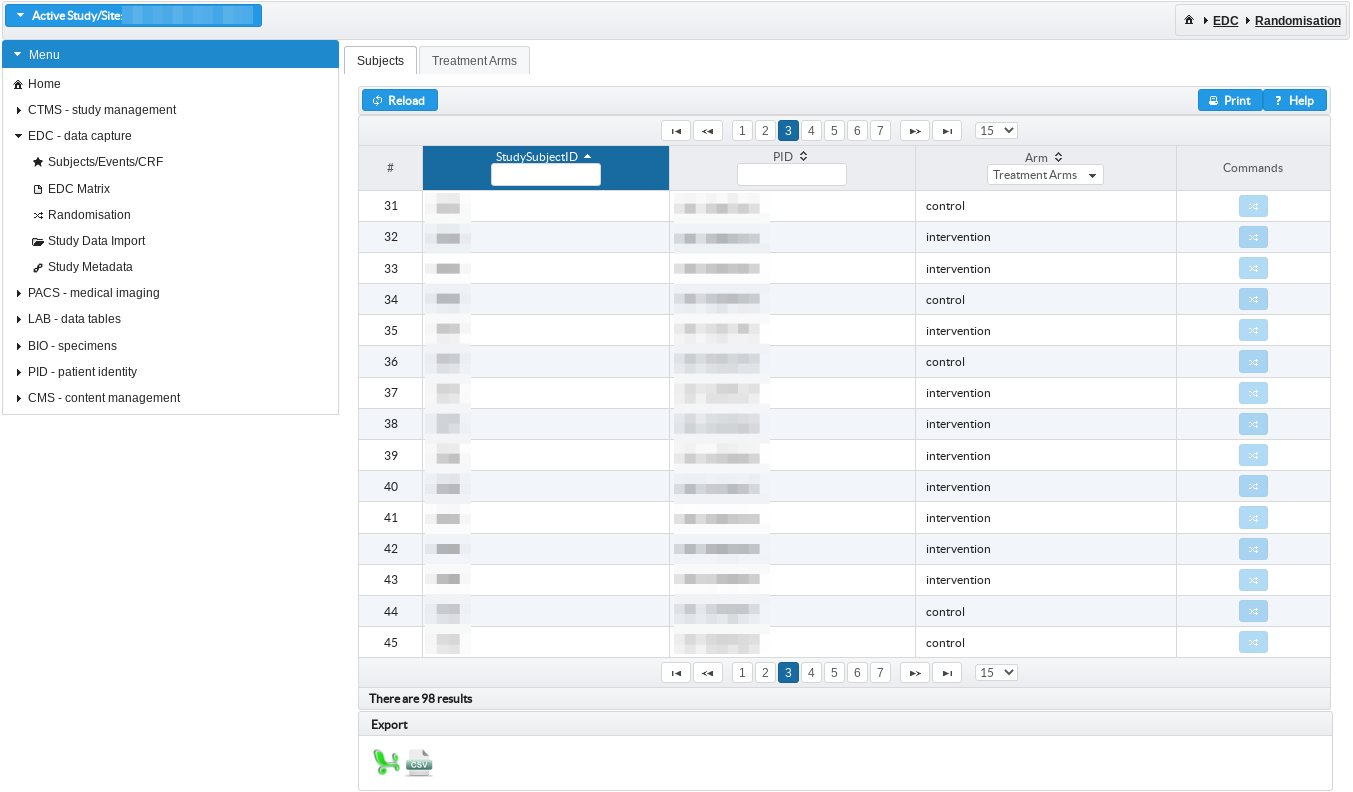
Randomisation of study subjects across treatment arms
Studies can be setup as randomised trials using permuted block randomisation with possibility to configure dichotomous stratification criteria and variable block size.
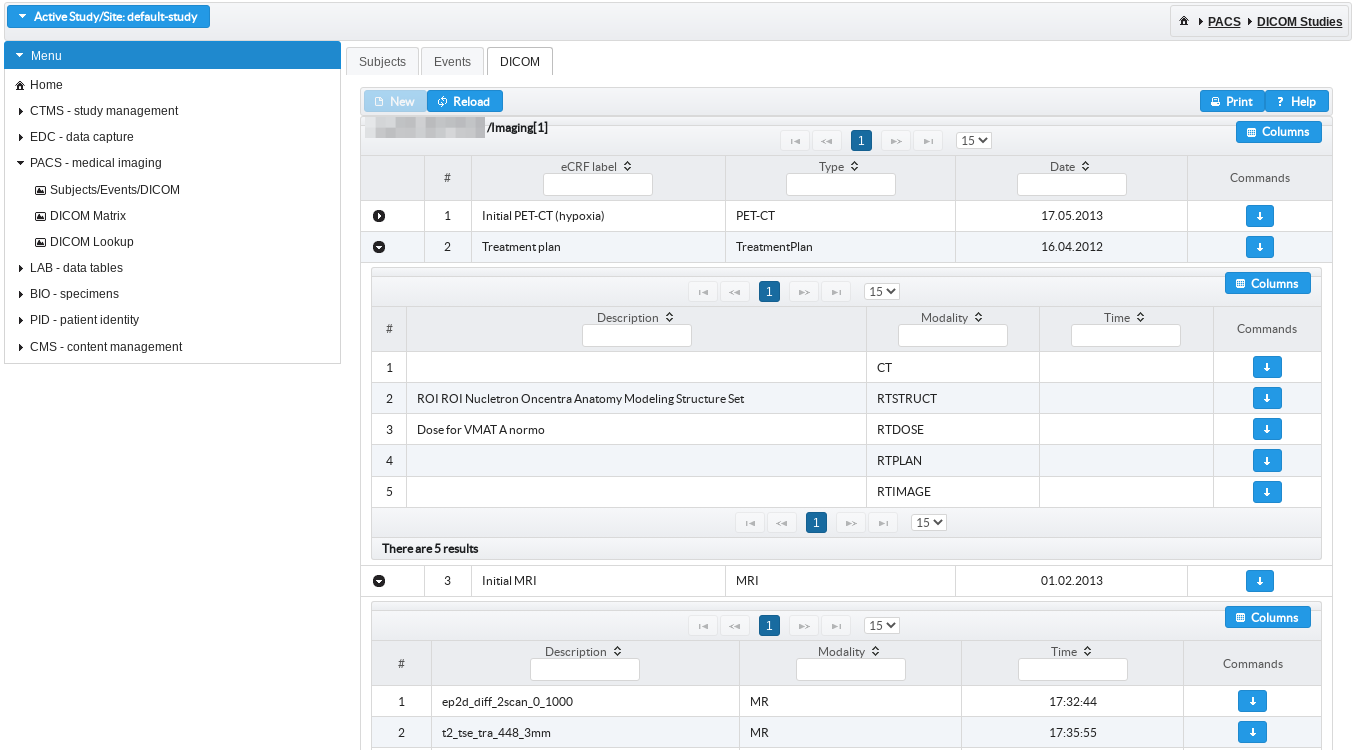
Linked medical imaging and treatment plans
Medical imaging (DICOM) and treatment planning (DICOM-RT) data can be de-identified on site and sent into upload slots linked with specific study events as defined in study protocol.
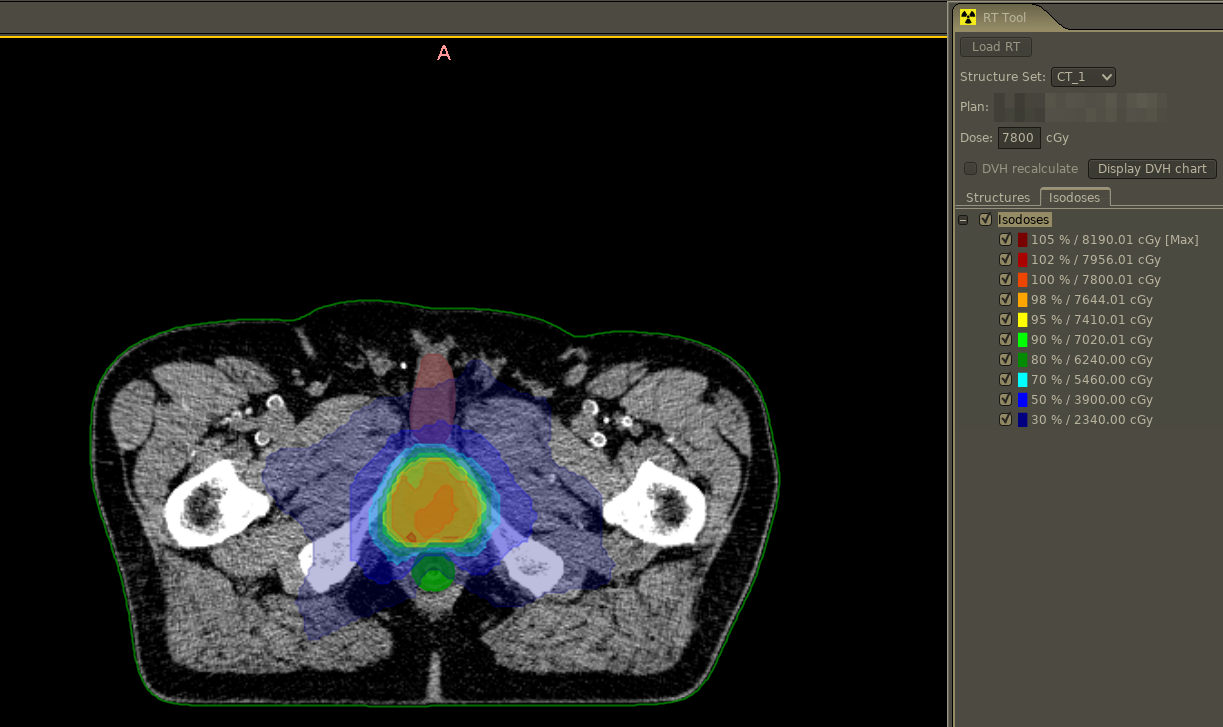
Standalone DICOM viewer with DICOM-RT plugin
Radiotherapy treatment plans can be loaded via standalone viewer with DICOM-RT support that renders de-liniated structures as well as calculated iso dose levels.
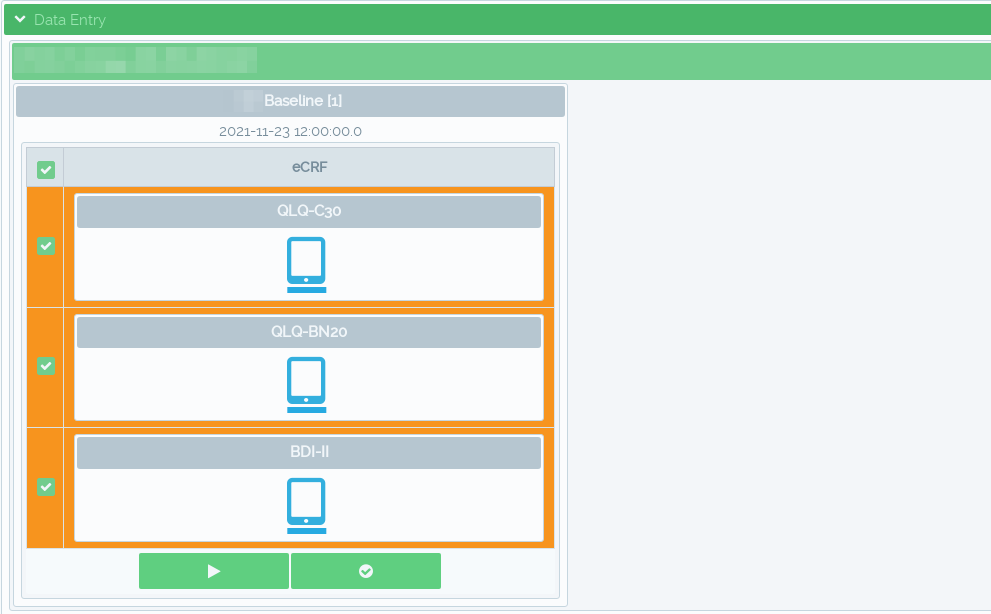
Patient reported outcomes (ePRO) during clinical patient visits
Using the mobile component the study personnel can use tablet interface to load special forms for direct patient data entry such as EORTC quality of life questionnaires.